Whether you’re working in small animal general practice, or are currently in veterinary emergency, GDV in dogs (Gastric Dilatation and Volvulus) is a pathological disease that always requires immediate intervention. This is due to its vast effects on the cardiovascular and respiratory system, coagulation cascade, spleen and alimentary tract. Without swift intervention, GDV in dogs can prove fatal, with patients requiring urgent veterinary treatment immediately upon presentation of clinical signs.
This disease process is commonly associated with a post-prandial state, as it is suggested that in deep chested dogs, eructation is prevented (dilation), as well as providing room for a pendulous effect leading to gastric torsion (volvulus). Consequently, GDV in dogs is seen in certain breeds that are over-represented, including the Great Dane, German Shepherd Dog, Labrador Retriever/Golden Retriever, Malamute and Weimeraner. It is important to note, gastric dilatation is not necessarily enough to cause volvulus, and volvulus can occur without dilatation.
Why does GDV in dogs occur?
In a normal anatomical state, the pylorus of the stomach normally exists on the right cranial side of the abdomen. Though with GDV, the pylorus moves ventrally across to the left side (clockwise direction, 95% cases), ‘kinking’ the oesophagus to prevent any chance of eructation. This is normally 180 degree torsion, however, torsions can exist between 90 – 360 degrees. Other ‘counter-clockwise’ directions can occur (though are seen in only 5% of cases) and reach a maximum of 90 degrees rotation.
GDV in dogs is also normally associated with a post-prandial state, especially after a large meal, though an underlying pathological outflow obstruction (functional or mechanical) also exists. Regardless of the rotation direction, cardiovascular stabilisation and manual derotation stands as the only means of treatment. Failure to perform either of these two adequately and efficiently will ultimately result in death of the patient.
Pathophysiology of GDV in Dogs
The single most important aspect associated with the pathophysiology of GDV is the compromise to global blood flow. Varying levels of shock are recognised in GDV, and it is vital to understand the pathophysiology to improve survival outcomes. The main organs and systems affected are summarised below.
Cardiovascular
Severe gastric distension increases intra-abdominal pressure, collapsing low pressure vessels such as the caudal vena cava, splanchnic veins and the portal vein. There is reduced venous return, leading to reduced ventricular filling and poor cardiac output, resulting in poor tissue and organ perfusion. Myocardial ischaemia also develops as coronary blood flow is reduced by 50%, leading to ventricular arrhythmias. Reduced portal circulation worsens venous congestion of abdominal splanchnic viscera and contributes to shock.
Respiratory
Pressure is placed on the diaphragm from a distended stomach, leading to a significantly reduced tidal volume. In combination with poor venous return and cardiac output, carbon dioxide is exhaled inefficiently, contributing to a respiratory acidosis. Due to the high volume of alimentary tract secretions, aspiration pneumonia can also develop (and is anecdotally noted in approximately 30% of patients at AEA).
Gastrointestinal
Distension of the stomach compromises blood flow to the gastric wall vasculature and stretches gastric arteries. In combination with a poor cardiac output, gastric necrosis develops. Serosal haemorrhage and oedema initially escalates in the fundus and extends to the stomach body and the distal oesophagus. Thinning of the gastric wall and development of oedema increases risk of bacterial translocation and development of septicaemia. This develops sub-acutely and its affects are normally seen post-operatively.
Spleen
As the splenic artery arises from the coeliac artery, venous thrombosis and arterial infarctions are commonly seen as this is stretched from gastric volvulus, causing splenic necrosis. If the spleen still appears necrotic following derotation of great vessels and the stomach, a splenectomy is performed. Although the hepatic artery arises from the coeliac artery as well, the liver is minimally affected due to arterial anastomoses.
Haemodynamics
As a result of gastric artery rupture, organ and tissue oedema, poor venous return, and global hypoxia from poor peripheral perfusion, cardiovascular shock and collapse become the main reasons for death in patients with GDV. Obstructive shock from abdominal main vessel collapse, hypovolaemic shock from blood and lymph loss, and global hypoxia from poor cardiac output make up the main components. In response to poor cardiac output and oxygen delivery, anaerobic metabolism occurs and lactate is formed as the byproduct causing a metabolic acidosis.
Lactate has been evaluated extensively as a measure of survivability and understanding gastric necrosis in GDV. Despite the results, patients with gastric necrosis can still have a low lactate and patients with a high lactate can have no gastric necrosis, suggesting there are multiple reasons why lactate might be elevated; it should not be considered a specific indicator of gastric necrosis.
Coagulation
There are a number of primary and secondary insults to the patient (Two-Hit Theory) with GDV that development of Systemic Inflammatory Response Syndrome (SIRS) and consequently Disseminated Intravascular Coagulation (DIC) are seen relatively commonly. Serosal oedema, macro and micro thromboses, septicaemia, global hypoxia, and surgical intervention are a select few that contribute to development of DIC.
Precise and efficient advice to owners prior to presentation, including treatment pre-operatively and intraoperatively is vital to reduce its incidence. Frequent monitoring is required post-operatively to act rapidly and eliminate development of Multi-Organ Dysfunction Syndrome (MODS).

Diagnosis of GDV in Dogs
The signs of GDV can be subtle at first, and early recognition and intervention are essential to improve your patient’s chances of survival. As a skilled veterinary surgeon, it is important to have a thorough understanding of the clinical signs and diagnostic methods to confidently diagnose dogs presenting with signs of GDV. Some of the most common clinical signs of GDV include:
- Distended and firm abdomen
- Salivating excessively
- Non-productive regurgitating/retching
- Panting and restless
- Muddy to Pale pink MM
- Prolonged CRT
Diagnostic Imaging
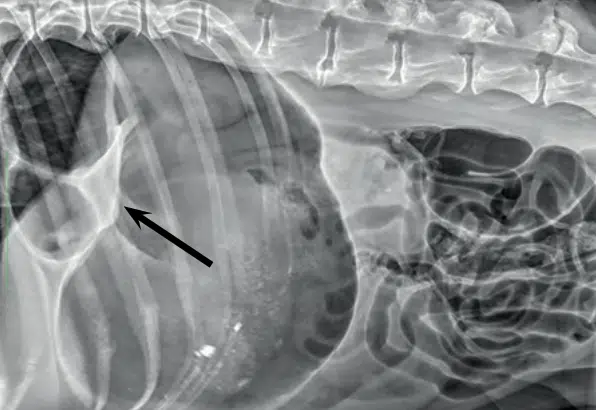
Radiography, specifically a right lateral radiograph of the abdomen, is required to determine a diagnosis. Thoracic radiographs are also helpful to determine the presence of aspiration pneumonia.
Typically, the pylorus is displaced dorsally and partly cranially, as most cases are attributed to 180 degree torsion. This is often accompanied by compartmentalisation of the stomach, as is indicated by the arrow in the radiograph pictured (Humm & Barfield, 2017).
Orthogonal views should also be considered if there is any consideration of non-surgical treatment. If the pylorus is on the left of midline and dorsally displaced, this is pathognomonic for GDV. However, cases of 360 degree rotations may be challenging to diagnose even with radiography.
Electrolytes
Historically, lactate levels >9mmol/L have been associated with a poorer prognosis than patients with normal lactate, but this should not be considered specific for gastric necrosis. Global hypoxia associated with shock also contributes to hyperlactataemia in GDV patients. Within 80 – 100 mins, the prognosis is improved provided lactate is reduced by >42% of presenting value, <6.4mmol/L and reduced by at least 4mmol/L units from the presenting value.
Hypokalemia may also be seen – acidosis causes potassium to move intracellularly, including sequestration in the stomach lumen, as well as 3rd space fluid loss. Hypoglycemia should also be monitored closely for, as this can be sequelae to septicaemia (bacterial metabolism) vs anaerobic metabolism (higher cellular demand for glucose to generate only 2x ATP molecules).
PCV/TS/Coagulation
Evidence of anaemia maybe present from rupture of gastric arteries and splenic vessels. This can be determined through a PCV or CBC. Ideally, an ACT +/- PT/APTT should also be performed due to risk of DIC and monitored both pre and post-operatively.

Treatment of GDV in Dogs
Although surgery and derotation is the mainstay of treatment of GDV in dogs, there are several more urgent steps required before considering surgery. This initial treatment involves several key steps that should be followed in each patient in order to stabilise and prepare the patient for surgery if necessary.
- Step 1: Oxygen
- Provide flow-by or mask oxygen
- Step 2: IV access
- Immediately place an IV catheter (14 – 18G) or 2 if possible
- Collect blood for electrolytes, lactate and coagulation (ACT vs PT/APTT) and biochemistry in high-risk patients
- Repeat tests within 80-100 mins (lactate movement can dictate prognosis and treatment goals)
- Step 3: Analgesia/Sedation
- Methadone 0.3mg/kg IM on admission
- +/- Fentanyl Bolus 2-5ug/kg q15 min until CRI established @ 3-5ug/kg/hr if going to surgery
- +/- Diazepam 0.2mg/kg IV
- Step 4: Fluid therapy
- Bolus crystalloid (pressure bag) in aliquots of 20ml/kg to effect
- +/- Hypertonic Saline @ 2-4mL/kg (C2)
- +/- Colloid @ 5-10mL/kg if fails to respond to crystalloid resuscitation and remains hypotensive
- Step 5: Decompression
- Pass a tube (small lumen to pass through stenotic oesophageal sphincter and remove gas only)
- Trocharisation can be performed under aseptic technique (R Lat, most upper tympanic region)
- Can worsen arrhythmias and SIRS/DIC due to reperfusion injury (release endotoxins and metabolites)
- Step 6: Imaging
- R Lat radiograph to confirm GD vs GDV (patient must be stabilised first)
GDV Surgery and Anaesthesia
This is the mainstay of treating GDV in dogs, as surgery to assess and derotate the stomach will determine overall prognosis for your patient. Though prior to and during surgery, several other components of treatment must be considered, particularly anaesthesia and medical management, including:
- Anaesthetic induction
- Alfaxalone 1-4mg/kg IV
- Propofol 2-8mg/kg IV
- Anaesthetic maintenance
- Restrict isoflurane gas to 1-2% maximum due to hypotensive effects. Utilise fentanyl CRI at higher doses. Patient may also require manual ventilation. Keep FiO2 100% at 1-2L/min
- Antibiotics
- Cephazolin 22mg/kg q2 hours until surgery completed
- Ongoing antibiotic coverage is not indicated unless evidence of mucosal damage and risk of bacterial translocation
- Gastric protectants
- Proton pump inhibitor: Omeprazole 1mg/kg IV q24hrs or Pantoprazole 1mg/kg IV q24hrs (C1)
GDV Surgery Method (Derotation and Gastropexy)
- Pass a well lubricated stomach tube once anaesthetized with the assistance of the surgeon preferably to prevent gastric perforation if gastric necrosis is present
- Assess direction of rotation through duodenal, omentum and oesophageal location
- Clockwise: duodenum is diagonal and ventral towards left side with omentum draped over dorsal surface of stomach (ie: omentum will appear ventral on entering the abdomen)
- Counter-clockwise: omentum is not present, feel oesophageal rotation direction at hiatus
- Derotate stomach
- Elevate pylorus towards ventral midline and depress fundus (clockwise). Opposite direction for counter-clockwise rotation
- Assess gastrosplenic ligament is not torsed
- Assess degree of gastric +/- oesophageal necrosis
- Resect necrotic gastric wall using autosuture (TA90 – preferred) or perform partial gastrectomy and close in 2 layers with synthetic absorbable suture
- NB: If gastrectomy requires >50% resection and/or there is oesophageal necrosis = Euthanasia
- Assess presence of splenic necrosis and arterial/venous thomboses (perform splenectomy if indicated)
- Empty stomach contents via oral tube and assess pyloric region for evidence of obstruction
- Only ever enter the stomach to remove a foreign object. Bones should be digested in time
- PEG tubes or feeding tubes are NOT indicated due to poor healing
- Perform Incisional Gastropexy
- 5 – 7cm incision in the seromuscular layer of the antrum midway between the greater and lesser curvatures of the stomach. The incision is then held up to the right lateral abdominal wall, just caudal to and parallel to the last rib
- Align with internal abdominal wall and make an incision through the peritoneum and transverse abdominus/internal fascia of the abdominal musculature.
- Pexy the stomach to the abdominal wall in a single continuous pattern using 0-PDS (tapered needle)
- Place Jackson-Pratt drain if concerned regarding gastric necrosis
- Close the abdomen as required in 3-layer technique
- Kink and remove stomach tube, swab larynx/pharynx
- Place nasogastric tube to allow continued gas release from stomach and to allow feeding
To access a free step-by-step guide to GDV diagnosis, management, and surgery, simply log in to VetAPedia and click the button below to view our detailed webinar on Surgical Emergencies with Dr Deeanna Ho.
Post-Operative Management of GDV in Dogs
Once surgical derotation of the stomach has been performed, focus on post-operative management is essential for a successful recovery. This requires on average 3 – 5 days of close hospital care, with costs while hospitalised varying between approximately $5000 – 8000. This is due to various factors required for post-operative treatment, which are detailed below:
- Fluid therapy
- Rate and crystalloid type based on patient assessment (typically 5-20ml/kg/hr)
- Higher fluid rates maybe required due to fluid 3 rd spacing into the peritoneum or gastrointestinal tract (likely a result of inflammation and hypoproteinemia)
- Analgesia
- Fentanyl CRI @ 2-5ug/kg/hr. Attempt to wean first due to complications with GIT status
- +/- Lignocaine IV bolus of 2mg/kg, then CRI at 2-3mg/kg/hr over first 24hrs post-surgery at least
- +/- Tramadol 2-4mg/kg q8-12hrs IV
- Prokinetics
- Metoclopramide 0.5mg/kg IV bolus, followed by CRI at max rate 0.08mg/kg/hr
- Anti-emetics
- Maropitant 1mg/kg SC q24hrs – maximum dose of 5 days
- Diagnostic tests
- Coagulation Screening @ 12-24hrs post-operatively due to risk of DIC
- Biochemistry @ 24hrs post-operatively due to risk of MODS
- PCV/TS to monitor for haemorrhaging/bleeding
- Electrolytes q4-8hrs within first 12-24hrs, then extend to q12-24hrs if improving. Hypokalemia potentiates ileus, gastroparesis, and cardiac arrhythmias if not treated
- Nutrition
- Lectade @ 1mL/kg CRI through NG tube for 12 hours then switch to Ensure vs Pediasure if anorexic at 1/3 RER. If tolerating well, increase by 1/3 every 8-12hrs
Prognosis and Risk Factors
As with any emergency, prognosis can vary significantly based on time to presentation, severity, and individual patient factors. This is especially true for cases of GDV in dogs, where swift intervention will often be life-saving, but heavily dependent on the length of clinical signs. In general, prognosis and risk factors are the following:
- Survival to discharge is reported at approximately 85% (15% mortality)
- Survival to discharge for dogs + partial gastrectomy is 70-74% (26-30% mortality)
- Recurrence rates for GDV following initial gastropexy is <5%
- Partial gastrectomy combined with splenectomy, hypotension, peritonitis, sepsis and DIC worsen prognosis
- Clinical signs existing for >6hrs prior to presentation are more prone to having partial gastrectomy, splenectomy or cardiac arrhythmias making their prognosis worse
- Lactate prognostic indicators
- Is used as an indicator of survival and not gastric necrosis
- Reduce by 50% within a 12hr period. Failure to reduce level has shown increased mortality
- Reduce by >42% within 80-100mins has improved survival rate
- Reduced to <6.4mmol/L and by >4mmol/L units has an improved survival rate
- Level of >9mmol/L on presentation has a worse prognosis
- Previous splenectomy is not associated with increased risk of developing GDV
Every case is individual, but having an idea of these key prognostic indicators will allow you and your client to be more informed both before and after treatment.
Cases of GDV in dogs can be challenging, even for veterinary professionals who are faced with them regularly. But with appropriate preparation and knowledge of diagnostics, treatment, and post-op management, these can be satisfying and life-saving cases.
So to ensure you’re fully prepared and equipped if a GDV presents in your clinic, be sure to download our full GDV protocol to easily keep in your clinic for quick reference and explore more of our patient care articles.